Biobank
ICVS / Resources & Facilities
Mission
MinhoMedBiobank’s mission is to mobilize the synergies of the School of Medicine and its associated laboratory, the Life and Health Sciences Research Institute (ICVS), its partner hospitals, primary healthcare units in the region, the Academic Clinical Centre (2CA-Braga), and the P5 Digital Medical Centre to build a sustainable regional coordination system that supports and promotes translational and clinical research. This infrastructure aims to foster basic, clinical, and translational biomedical research, with the goal of contributing to a deeper understanding of disease mechanisms, the development of new diagnostic tests and therapeutic strategies, and the support of postgraduate scientific training for physicians and other healthcare professionals.
MinhoMedBiobank consists of a large collection of human biological samples (serum, plasma, cells, cerebrospinal fluid, urine, saliva, DNA, and tissues) and collections of digital imaging data, both accompanied by associated clinical and lifestyle information.
Specific objectives of MinhoMedBiobank:
- Ensure the continuous collection of a wide range of human biological samples associated with relevant clinical information, including clinical imaging data when available.
- Ensure the quality of stored material through standardized procedures in accordance with applicable national and international regulations, standards, and laws.
- Monitor the use of samples under the supervision of the MinhoMedBiobank Scientific Committee.
- Integrate the National Biobank Platform and promote its inclusion in the European network, the Biobanking and BioMolecular Resources Research Infrastructure (BBMRI).
Regulatory and Ethical Framework
All procedures were developed in accordance with best technical practices and applicable legal and ethical guidelines in the field of biobanking, namely:
- OECD: Best Practice Guidelines for Biological Resource Centres;
- ISO 20387:2018 Biotechnology – Biobanking: General requirements for biobanking;
- Law No. 58/2019 of 8 August – Personal Data Protection Law; Article 6(1)(e) GDPR – Public interest and Article 9(2)(a) and (j) GDPR – Explicit consent and scientific research;
- Regulation (EU) 2016/679 of 27 April on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, repealing Directive 95/46/EC (General Data Protection Regulation – GDPR);
- Law No. 12/2005 of 26 January – Law on personal genetic information and health information;
- Favourable opinion from the Ethics Committee (Ethics Committee of the University of Minho).
Equipment and Technologies
The MinhoMedBiobank is equipped with all the necessary infrastructure to ensure quality in storage facilities, pre-analytical processing tools, data recording, and information sharing, namely:
- Monitored –80°C freezers;
- –80°C backup freezer;
- Rapid freezing unit (liquid nitrogen transport container);
- BSL-2 biosafety laboratory;
- LIMS (LabWare) system for sample and data management;
- 2D barcode reading equipment;
- Physical and logical access control systems.
How to Use the MinhoMedBiobank Service
The MinhoMedBiobank provides services for the collection, processing, storage, and distribution of human biological samples, as well as associated clinical and medical imaging data, for scientific research purposes.
- Who can use the service
The service is available to researchers and research teams from academia and partner institutions, within the scope of duly approved clinical, translational, or biomedical research projects.
- Sample submission to the Biobank
Samples may enter the MinhoMedBiobank through two pathways:
- Within research projects: Sample collection is the responsibility of the researcher, and the collection protocol must be previously agreed upon with the Biobank. Samples are delivered to or collected by the Biobank, as defined.
- Through systematic collection by the Biobank: The Biobank establishes agreements with hospital services or primary healthcare units for the systematic collection of specific types of biological samples.
In all cases, valid informed consent and relevant clinical information are mandatory.
- Processing and storage
After entering the Biobank:
- Each sample is coded/pseudonymised;
- Samples are processed according to standard operating procedures (SOPs);
- Associated information is recorded in the LIMS;
- Samples are stored under controlled and secure conditions.
- Access to and release of samples
Access to samples and data requires:
- Submission of a formal request;
- Prior approval by an Ethics Committee;
- Validation by the MinhoMedBiobank Scientific Committee.
Samples may be subject to different levels of access restriction, defined at the time of their integration into the Biobank.
- Additional services
The MinhoMedBiobank offers optional sample processing services, subject to prior agreement and according to specific procedures.
- Sample retention
Samples are stored for a maximum period of 10 years, with periodic reviews of their relevance and conditions of use, in accordance with established rules.
- Ethical aspects and data protection
All MinhoMedBiobank activities comply with applicable national and European legislation, particularly the GDPR, ensuring the confidentiality, security, and ethical use of samples and associated data. The MinhoMedBiobank received favourable opinion from the Ethics Committee (Ethics Committee of the University of Minho).
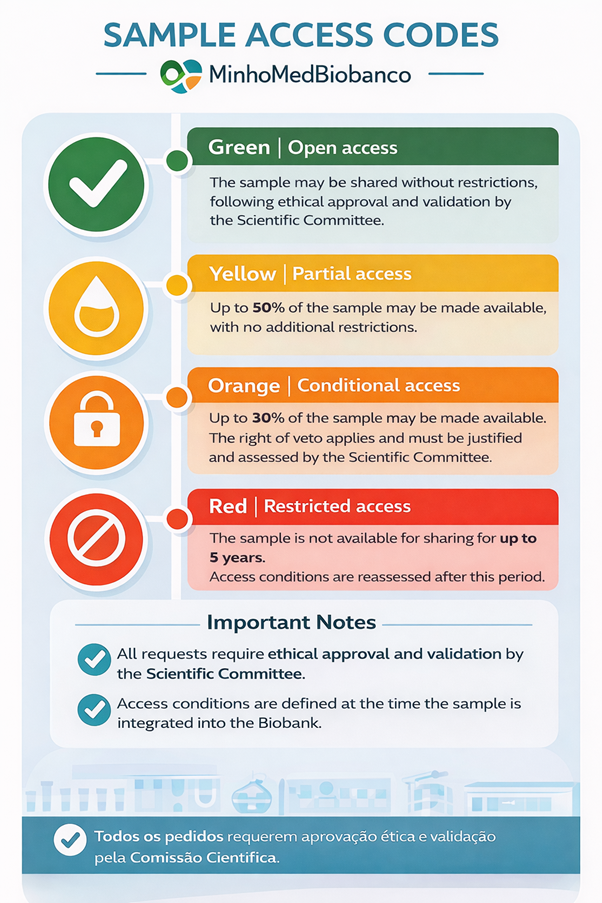
Color Codes for Sample Access – MinhoMedBiobank
|
Color |
Access Level |
Description |
|
🟢 Green |
Open access |
The sample may be shared without restrictions, following ethical approval and validation by the Scientific Committee. |
|
🟡 Yellow |
Partial access |
The depositing group makes up to 50% of the sample available for sharing, with no additional restrictions. |
|
🟠 Orange |
Conditional access |
The depositing group makes up to 30% of the sample available and retains the right of veto over access requests, which must be duly justified during a Scientific Committee meeting. |
|
🔴 Red |
Restricted access |
The sample is not available for sharing for a defined period (up to 5 years). After this period, the access level is reassessed. |
Important Notes
- All access requests are subject to ethical approval and validation by the MinhoMedBiobanco Scientific Committee.
- Access conditions are defined at the time the sample is integrated into the Biobank and formalized through a written agreement.
- Sample release implies the minimum sharing of clinical information, as defined by the depositing group.



Contact us
Phone: +351 253 604 967
Fax: +351 253 604 809
Email: icvs.sec@med.uminho.pt
Address
Life and Health Sciences
Research Institute (ICVS)
School of Medicine,
University of Minho,
Campus de Gualtar
4710-057 Braga
Portugal

Copyright ©2025 ICVS. All Rights Reserved. Developed by TCIT



Copyright ©2025 ICVS. All Rights Reserved. Developed by TCIT
Address
Life and Health Sciences
Research Institute (ICVS)
School of Medicine,
University of Minho,
Campus de Gualtar
4710-057 Braga
Portugal



Copyright ©2025 ICVS. All Rights Reserved
Address
Life and Health Sciences
Research Institute (ICVS)
School of Medicine,
University of Minho,
Campus de Gualtar
4710-057 Braga
Portugal





