Translational Neurogenetics
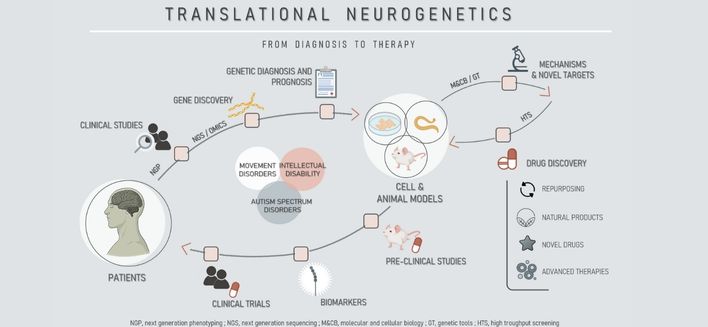
The goal of our research is to understand the genetic basis of human neurological diseases, using molecular genetics, genomics, transcriptomics, proteomics, cell biology, electrophysiology and behavioral analysis, and to apply this knowledge to the clinical context. We study neurodegenerative and neurodevelopmental disorders, from gene identification in human patient cohorts to mechanistic studies and preclinical drug testing. For this we develop disease models using cultured cells, C. elegans and mice, which, once validated, can be used to search for therapeutic targets and test the efficacy of therapeutic strategies – based on novel synthetic molecules, natural products and repurposed drugs.
Within our studies of disease mechanisms, a topic of particular relevance is the regulation of protein homeostasis within neurons, through the action of a network of protein folding and protein degradation promoting molecules. The function of these molecules in neuronal cells is of interest to us from a fundamental science perspective but also as potential drug targets. We have acquired expertise and developed relevant tools for the study of these pathways, from chaperones to the ubiquitin-proteasome system, and autophagy, as well as to address degradation-unrelated ubiquitin signalling in the nervous system. We are also working on new small-molecule based therapeutic modalities for neurodegenerative diseases that harness these endogenous targeted protein degradation mechanisms or target selected protein-protein interactions.
Team Members


Andreia Teixeira-Castro

Sara Duarte-Silva

Cláudia Falcão-Reis

Jorge Diogo Da Silva

Maria Lopes de Almeida

Bruno Almeida

Joana Pereira-Sousa

Marta Daniela Costa

Stéphanie Oliveira

Cármen Vieira

Cidália Pereira

Daniela Cunha-Garcia

Daniela Monteiro-Fernandes

Daniela Vilasboas-Campos

Joana Sofia Correia

Jorge H. Fernandes

Liliana Meireles-Costa

Sara Ramalhosa Guerreiro

Isabella França

Lídia Nunes-Carvalho

Patrícia Araújo

Rúben V. Freitas

Bruna Ferreira-Lomba

Rita Fernandes
Team Members

Patricia Maciel
Principal Investigator
Team Coordinator

Andreia Teixeira-Castro
Assistant Researcher

Sara Duarte-Silva
Assistant Researcher

Cláudia Falcão-Reis
Clinician-Researcher

Jorge Diogo Da Silva
Clinician-Researcher

Maria Lopes de Almeida
Clinician-Researcher

Bruno Almeida
Postdoctoral Researcher

Joana Pereira-Sousa
Postdoctoral Researcher

Marta Daniela Costa
Postdoctoral Researcher

Stéphanie Oliveira
Postdoctoral Researcher

Cármen Vieira
PhD Student

Daniela Cunha-Garcia
PhD Student

Daniela Monteiro-Fernandes
PhD Student

Daniela Vilasboas-Campos
PhD Student

Joana Sofia Correia
PhD Student

Jorge H. Fernandes
PhD Student

Liliana Meireles-Costa
PhD Student

Sara Ramalhosa Guerreiro
PhD Student

Isabella França
MSc Student

João Barbosa
MSc Student
Projects
- Patricia Maciel
- Our multidisciplinary research team aims at dentifying and validating novel genetic causes of neurodevelopmental disorders in human patients, taking advantage of clinically well characterized patient cohorts and…
- Patricia Maciel
- Departing from previous work in which we developped cellular and animal models of Machado-Joseph disease, a neurodegenerative disorder caused by expansion of a polyglutamine tract in the protein ataxin-3 (ATXN3)…
- Patricia Maciel
- Our goal is to uncover mechanisms of disease and to test potential therapeutic approaches for disorders of human neurodevelopment, including intellectual disability (ID) and autism, but also early onset movement disorders…
- Andreia Teixeira-Castro
- This project addresses an unmet medical need- the lack of effective treatment for any of the aging-associated neurodegenerative diseases. Due to the worldwide aging of the population, by 2050 it is expected that over 135 million people…
Success Story
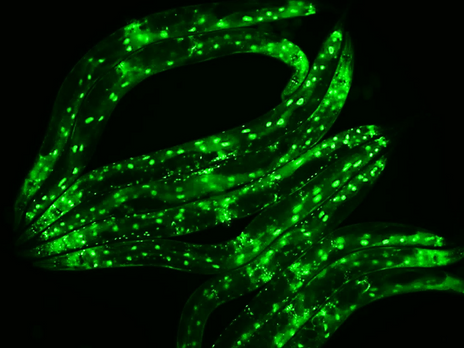
Our team has a strong and internationally recognized scientific expertise in Machado-Joseph disease – a rare neurodegenerative disease within the group of polyglutamine diseases– and in proteostasis, related to normal aging and to aging-related neurodegenerative disorders. We have developed highly relevant animal models of this disease in C. elegans and mouse, that mimick the core features of the human disease, from pathology to motor phenotype. We optimized a setup for medium-throughput drug screening based on the C. elegans model that has allowed the discovery of highly relevant therapeutic candidates and provided novel clues to disease pathogenesis mechanisms. Three of the molecules identified using this approach – citalopram/escitalopram, creatine and tauroursodeoxycholic acid (TUDCA) – are easily translatable to the clinical context, in a drug repurposing approach, given their known safety, and thus may allow closing this virtuous circle with impact on patient care.
Selected Research Outputs
Fu, J. M., F. K. Satterstrom, M. Peng, H. Brand, R. L. Collins, S. Dong, B. Wamsley, L. Klei, L. Wang, S. P. Hao, C. R. Stevens, C. Cusick, M. Babadi, E. Banks, B. Collins, S. Dodge, S. B. Gabriel, L. Gauthier, S. K. Lee, L. Liang, A. Ljungdahl, B. Mahjani, L. Sloofman, A. N. Smirnov, M. Barbosa, C. Betancur, A. Brusco, B. H. Y. Chung, E. H. Cook, M. L. Cuccaro, E. Domenici, G. B. Ferrero, J. J. Gargus, G. E. Herman, I. Hertz-Picciotto, P. Maciel, D. S. Manoach, M. R. Passos-Bueno, A. M. Persico, A. Renieri, J. S. Sutcliffe, F. Tassone, E. Trabetti, G. Campos, S. Cardaropoli, D. Carli, M. C. Y. Chan, C. Fallerini, E. Giorgio, A. C. Girardi, E. Hansen-Kiss, S. L. Lee, C. Lintas, Y. Ludena, R. Nguyen, L. Pavinato, M. Pericak-Vance, I. N. Pessah, R. J. Schmidt, M. Smith, C. I. S. Costa, S. Trajkova, J. Y. T. Wang, M. H. C. Yu, B. Aleksic, M. Artomov, E. Benetti, M. Biscaldi-Schafer, A. D. Børglum, A. Carracedo, A. G. Chiocchetti, H. Coon, R. N. Doan, M. Fernández-Prieto, C. M. Freitag, S. Gerges, S. Guter, D. M. Hougaard, C. M. Hultman, S. Jacob, M. Kaartinen, A. Kolevzon, I. Kushima, T. Lehtimäki, C. L. Rizzo, N. Maltman, M. Manara, G. Meiri, I. Menashe, J. Miller, N. Minshew, M. Mosconi, N. Ozaki, A. Palotie, M. Parellada, K. Puura, A. Reichenberg, S. Sandin, S. W. Scherer, S. Schlitt, L. Schmitt, K. Schneider-Momm, P. M. Siper, P. Suren, J. A. Sweeney, K. Teufel, M. del Pilar Trelles, L. A. Weiss, R. Yuen, D. J. Cutler, S. De Rubeis, J. D. Buxbaum, M. J. Daly, B. Devlin, K. Roeder, S. J. Sanders, M. E. Talkowski, C. The Autism Sequencing, G. Broad Institute Center for Common Disease and P.-B. C. i (2022). Rare coding variation provides insight into the genetic architecture and phenotypic context of autism. Nature Genetics, 54(9), 1320-1331. https://doi.org/10.1038/s41588-022-01104-0
Campos, A. B.*, Duarte-Silva, S.*, Fernandes, B., das Neves, S. P., Marques, F., Teixeira-Castro, A., Neves-Carvalho, A., Monteiro-Fernandes, D., Portugal, C. C., Socodato, R., Summavielle, T., Ambrósio, A. F., Relvas, J. B., & Maciel, P. (2022). Profiling Microglia in a Mouse Model of Machado-Joseph Disease. Biomedicines, 10(2), 237. https://doi.org/10.3390/biomedicines10020237
Jalles, A.*, Vieira, C.*, Pereira-Sousa, J., Vilasboas-Campos, D., Mota, A. F., Vasconcelos, S., Ferreira-Lomba, B., Costa, M. D., Da Silva, J. D., Maciel, P., & Teixeira-Castro, A. (2022). Aripiprazole Offsets Mutant ATXN3-Induced Motor Dysfunction by Targeting Dopamine D2 and Serotonin 1A and 2A Receptors in C. elegans. Biomedicines, 10(2), 370. https://doi.org/10.3390/biomedicines10020370
Pereira-Sousa, J., Ferreira-Lomba, B., Bellver-Sanchis, A., Vilasboas-Campos, D., Fernandes, J. H., Costa, M. D., Varney, M. A., Newman-Tancredi, A., Maciel, P., & Teixeira-Castro, A. (2021). Identification of the 5-HT1A serotonin receptor as a novel therapeutic target in a C. elegans model of Machado-Joseph disease. Neurobiology of Disease, 152, 105278. https://doi.org/10.1016/j.nbd.2021.105278
Correia, J. S.*, Neves-Carvalho, A.*, Mendes-Pinheiro, B.*, Pires, J., Teixeira, F. G., Lima, R., Monteiro, S., Silva, N. A., Soares-Cunha, C., Serra, S. C., Duarte-Silva, S., Teixeira-Castro, A., Salgado, A. J., & Maciel, P. (2021). Preclinical Assessment of Mesenchymal-Stem-Cell-Based Therapies in Spinocerebellar Ataxia Type 3. Biomedicines, 9(12), 1754. https://doi.org/10.3390/biomedicines9121754
Satterstrom, F. K., J. A. Kosmicki, J. Wang, M. S. Breen, S. De Rubeis, J. Y. An, M. Peng, R. Collins, J. Grove, L. Klei, C. Stevens, J. Reichert, M. S. Mulhern, M. Artomov, S. Gerges, B. Sheppard, X. Xu, A. Bhaduri, U. Norman, H. Brand, G. Schwartz, R. Nguyen, E. E. Guerrero, C. Dias, C. Betancur, E. H. Cook, L. Gallagher, M. Gill, J. S. Sutcliffe, A. Thurm, M. E. Zwick, A. D. Børglum, M. W. State, A. E. Cicek, M. E. Talkowski, D. J. Cutler, B. Devlin, S. J. Sanders, K. Roeder, M. J. Daly and J. D. Buxbaum (2020). "Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism." Cell 180(3): 568-584.e523.
Duarte-Silva, S., Neves-Carvalho, A., Soares-Cunha, C., Silva, J. M., Teixeira-Castro, A., Vieira, R., Silva-Fernandes, A., & Maciel, P. (2018). Neuroprotective Effects of Creatine in the CMVMJD135 Mouse Model of Spinocerebellar Ataxia Type 3. Movement disorders : official journal of the Movement Disorder Society, 33(5), 815–826. https://doi.org/10.1002/mds.27292
Teixeira-Castro, A.*, Jalles, A.*, Esteves, S.*, Kang, S., da Silva Santos, L., Silva-Fernandes, A., Neto, M. F., Brielmann, R. M., Bessa, C., Duarte-Silva, S., Miranda, A., Oliveira, S., Neves-Carvalho, A., Bessa, J., Summavielle, T., Silverman, R. B., Oliveira, P., Morimoto, R. I., & Maciel, P. (2015). Serotonergic signalling suppresses ataxin 3 aggregation and neurotoxicity in animal models of Machado-Joseph disease. Brain: a journal of neurology, 138(Pt 11), 3221–3237. https://doi.org/10.1093/brain/awv262
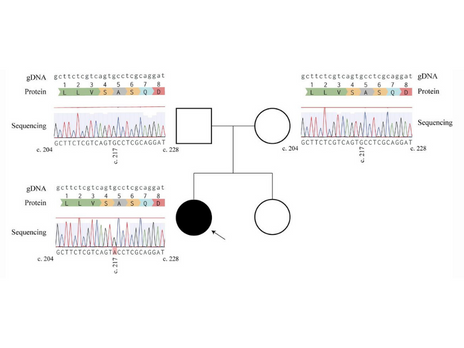
Lopes, F., Barbosa, M., Ameur, A., Soares, G., de Sá, J., Dias, A. I., Oliveira, G., Cabral, P., Temudo, T., Calado, E., Cruz, I. F., Vieira, J. P., Oliveira, R., Esteves, S., Sauer, S., Jonasson, I., Syvänen, A. C., Gyllensten, U., Pinto, D., & Maciel, P. (2016). Identification of novel genetic causes of Rett syndrome-like phenotypes. Journal of Medical Genetics, 53(3), 190–199. https://doi.org/10.1136/jmedgenet-2015-103568
Patent: “Citalopram or escitalopram, pharmaceutically acceptable salts thereof for use in the treatment of neurodegenerative diseases” to the University of Minho - EP16715099.4 (PM and ATC- Inventors)



Contact us
Phone: +351 253 604 967
Fax: +351 253 604 809
Email: icvs.sec@med.uminho.pt
Address
Life and Health Sciences
Research Institute (ICVS)
School of Medicine,
University of Minho,
Campus de Gualtar
4710-057 Braga
Portugal

Copyright ©2022 ICVS. All Rights Reserved



Copyright ©2022 ICVS. All Rights Reserved
Address
Life and Health Sciences
Research Institute (ICVS)
School of Medicine,
University of Minho,
Campus de Gualtar
4710-057 Braga
Portugal



Copyright ©2022 ICVS. All Rights Reserved
Address
Life and Health Sciences
Research Institute (ICVS)
School of Medicine,
University of Minho,
Campus de Gualtar
4710-057 Braga
Portugal