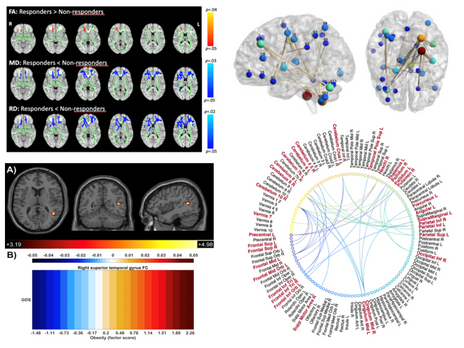
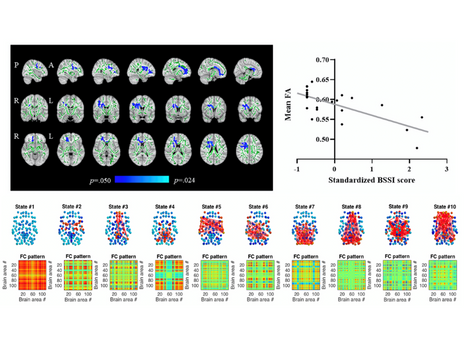
Brain Circuits and Neuron-glia Adaptations
The Brain Circuits and Neuron-Glia Adaptations (C2B) team studies the mechanisms underlying mood, cognition, and motivation in physiological and pathological contexts. Using state-of-the-art technological platforms, our team combines basic, translational, and clinical research via an academia-industry productive partnership.
Our team develops studies from molecules to cells (neurons, glia), to circuits (anatomic and functional), to behavior (normal and maladaptive), in order to understand the basis of mood, cognition, and motivation. The fundamental neuroscience studies developed by our team set the basis to better understand the pathophysiological mechanisms of neurological/neuropsychiatric disorders such as addiction, depression and Alzheimer’s disease, in both preclinical models and humans.
In parallel, our clinical research studies explore novel neuroimaging and molecular biomarkers and therapeutic approaches in Major Depression and Treatment Resistant Depression with observational neuroimaging studies in clinical populations and clinical trials with new antidepressant molecules while our recent translational work focuses on the biomarker potential of exosomes.
This bidirectional scientific pipeline between fundamental and clinical neuroscience constitutes the key element that will enable this team to achieve the ambitious objectives of translating our ground-breaking scientific results about the cellular and circuit brain machinery to the clinical context, developing innovative therapeutic strategies to be tested in real-world clinical populations and create value and intellectual property in partnership with strategic partners in the industry.
Scientific excellence, Clinical impact, Innovation, Outreach activities for the society
Team Members


Ana João Rodrigues

Ioannis Sotiropoulos

João Bessa

João Oliveira

Bárbara Coimbra

Carina Soares-Cunha

Joana Margarida Silva

Neide Vieira

Nuno Dinis Alves

João Vaz Silva

Patrícia Gomes

Leandro A. Aguiar

Tawan T. A. Carvalho

Teresa Canedo

Ana Monteiro-Pacheco

Ana Verónica Domingues

Andreia Vaz

Daniela Abreu

Gisela Armada

Inês Moreira Ribeiro

João F Viana

João Machado

Marcelina Węzik

Natacha Vieitas-Gaspar

Raquel Correia

Rita Vieira

Sara Barsanti

Tiago Silveira-Rosa

Alexandra Veiga

Ana Vale

Iara Pereira da Silva

José Duarte Dias

Luísa Ferraz

Samuel Alves

Ana Rita Dourado

Beatriz Barros dos Santos

Carlos Campos-Marques

Eduardo Teixeira

Gonçalo Martins Ferreira

Rita Nóbrega Amaral Martins
Team Members

Luisa Pinto
Principal Investigator
Team Coordinator

Ana João Rodrigues
Principal Investigator

João Bessa
Principal Investigator

João Oliveira
Principal Investigator

Ioannis Sotiropoulos
Principal Investigator

Bárbara Coimbra
Assistant Researcher

Carina Soares-Cunha
Assistant Researcher

João Vaz Silva
Clinician-Researcher

Neide Vieira
Assistant Researcher

Claire Terrier
Postdoctoral Researcher

Joana Margarida Silva
Postdoctoral Researcher

Leando A. Aguiar
Postdoctoral Researcher

Tawan T. A. Carvalho
Postdoctoral Researcher

Ana Verónica Domingues
PhD Student

Andreia Vaz
PhD Student

Daniela Abreu
PhD Student

Natacha Vieitas-Gaspar
PhD Student

Patrícia Gomes
PhD Student

Raquel Correia
PhD Student

Rita Vieira
PhD Student

Sara Barsanti
PhD Student

Tiago Silveira-Rosa
PhD Student

Ana Vale
MSc Student

Anabela Ferreira
MSc Student

Beatriz Barros dos Santos
MSc Student

Catarina Deseyve
MSc Student

Gonçalo Ferreira
MSc Student

Iara Pereira da Silva
MSc Student

Ricardo Bastos-Gonçalves
MSc Student

Rita Martins
MSc Student

Romeu Fernandes
MSc Student

Ana Vilaça-Ferreira
Research Technician

Carlos Campos-Marques
Research Technician

Marcelina Węzik
Research Technician

Rita Gaspar
Research Technician
Projects
- João Bessa
- Depression is a highly prevalent mood disorder that inflicts a heavy burden in depressed patients and a significant social and economic impact worldwide…
- Bárbara Coimbra
- The brain constantly integrates new sensory information, and associates environmental cues to outcomes, adjusting behavior to maximize reward and minimize unpleasant consequences. This process is critical for survival, and its dysregulation is a hallmark of…
- Ioannis Sotiropoulos
- Although cumulative evidence suggests a continuum between depression and AD, and stress is suggested to play a detrimental role in both diseases…
- João Oliveira
- Cognitive functioning is the intellectual activity that includes mental processes, such as, attention, learning and memory, executive function, verbal fluency, and working memory…
- Neide Vieira
- Our research main focus over the past years has been to understand the molecular mechanisms underlying proteostasis regulation and their relevance for physiological and pathological processes…
- Ana João Rodrigues
- Early life adversity can have long lasting consequences for the individual, increasing the vulnerability to develop neuropsychiatric disorders later in life…
- Luisa Pinto
- Depression affects around 16% of the world population and is the leading cause of disability worldwide. This means that around 300 million people are now living with depression…
- Luisa Pinto
- The adult mammalian brain harbors different forms of neural plasticity, ranging from neuronal synapto-dendritic rearrangements to the generation of new neuronal…
- João Oliveira
- Depressive disorders affect more than 280 million people worldwide and its pathophysiology is still poorly understood. Depressive behavior is characterized in humans…
- Ana João Rodrigues
- Since the moment we wake up, we are continuously flooded with sensory information of variable relevance. Therefore, our brains evolved to filter information…
- Carina Soares-Cunha
- Through evolution, animals gained the remarkable ability to respond with sub second precision to environmental stimuli and to learn to associate those with positive or negative outcomes…
- Ioannis Sotiropoulos
- Alzheimer’s disease (AD), the leading cause of dementia, affects nearly 44 million people worldwide and costs over $600 billion/year (1% of the world’s GDP)…
- João Bessa
- Depression is a leading cause of disability worldwide with a great personal, social, and economic impact. It is the most common disorder at the time of death by suicide, which is a major public health concern…
- Ioannis Sotiropoulos
- While Tau protein is originally described as a mainly axonal, microtubule-associated protein, accumulating evidence supports the multiple roles of Tau in different cell…
Success Story
Fundamental Neuroscience: Our team was awarded with an ERC Consolidator grant to investigate how valence is encoded in our brain and how this information leads to rewarding and aversive outcomes;
Clinical Research: We have currently running one Phase 1 Clinical Trial “A Randomised, Double-blind, Placebo-controlled Crossover EEG Study to Investigate the effect of GT-002 in Healthy Subjects” in 2CA-Braga – Industry Promoter: Gabather;
Entrepreneurship: We created one start-up company BNML – Behavioral & Molecular Lab, Lda, which uses advanced analytical methods to assess the therapeutic potential of new compounds at the behavioral, cellular, and molecular level, using animal models that mimic the symptoms of human disease.
Selected Research Outputs
Loureiro-Campos, E., Mateus-Pinheiro, A., Patrício, P., Soares-Cunha, C., Silva. J., Sardinha, V.M., Mendes-Pinheiro, B., Silveira-Rosa, T., Domingues, A.V., Rodrigues, A.J., Oliveira, J.F., Sousa, N., Alves, N.D.* and Pinto, L.* (2021). Constitutive AP2gamma deficiency reduces postnatal hippocampal neurogenesis, inducing anxious-like phenotype and memory impairments in juvenile mice that either persist or emerge during adulthood. eLife, 10:e70685. doi: 10.7554/eLife.70685.
Mateus-Pinheiro, A., Patrício, P., Alves, N.D., Martins-Macedo, J., Caetano, I., Silveira-Rosa, T., Araújo, B., Mateus-Pinheiro, M., Silva-Correia, J., Sardinha, V.M., Loureiro-Campos, E., Rodrigues, A.J., Oliveira, J.F., Bessa, J.M., Sousa, N., Pinto, L.* (2021). Hippocampal cytogenesis abrogation impairs inter-regional communication between the hippocampus and prefrontal cortex and promotes the time-dependent manifestation of emotional and cognitive deficits. Molecular Psychiatry, Sep 14. doi: 10.1038/s41380-021-01287-8
Coimbra B, Soares-Cunha C, Vasconcelos NAP, Domingues AV, Borges S, Sousa N*, Rodrigues AJ* (2019). Role of laterodorsal tegmentum projections to nucleus accumbens in reward-related behaviors. Nat Commun 10, 4138. https://doi.org/10.1038/s41467-019-11557-3
Soares-Cunha C, de Vasconcelos NAP, Coimbra B, Domingues AV, Silva JM, Loureiro-Campos E, Gaspar R, Sotiropoulos I, Sousa N*, Rodrigues AJ* (2019). Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol Psychiatry Dec;25(12):3241-3255. doi: 10.1038/s41380-019-0484-3.
Morais M, Patrício P, Mateus-Pinheiro A, Alves ND, Machado-Santos AR, Correia JS, Pereira J, Pinto L, Sousa N, Bessa JM*. (2017). The modulation of adult neuroplasticity is involved in the mood-improving actions of atypical antipsychotics in an animal model of depression. Transl Psychiatry. Jun 6;7(6):e1146. doi: 10.1038/tp.2017.120.
Vieira R, Coelho A, Reis J, Portugal-Nunes C, Magalhães R, Ferreira S, Moreira PS, Sousa N, Bessa JM*. (2021). White Matter Microstructure Alterations Associated With Paroxetine Treatment Response in Major Depression. Front Behav Neurosci. Jul 22;15:693109. doi: 10.3389/fnbeh.2021.693109.
Silva J, Rodrigues S, Sampaio-Marques B, Gomes P, Neves-Carvalho A, Dioli C, Soares-Cunha C, Takashima A, Ludovico P, Wolozin B, Sousa N, Sotiropoulos I*. (2019). Dysregulation of autophagy and Stress granule-related proteins in stress-driven Tau pathology. Cell Death Diff. Aug;26(8):1411-1427. doi: 10.1038/s41418-018-0217-1.
Monteiro-Fernandes D, Silva JM, Soares-Cunha C, Dalla C, Kokras N, François A, Billiras R, Bretin S, Sousa N, Sotiropoulos I*. (2020). Allosteric modulation of AMPA receptors counteracts Tau-related excitotoxic synaptic signaling and memory deficits in Stress and Aβ-evoked hippocampal pathology. Molecular Psychiatry May 28. doi: 10.1038/s41380-020-0794-5.
Guerra-Gomes S, Cunha-Garcia D, Marques Nascimento DS, Duarte-Silva S, Loureiro-Campos E, Morais Sardinha V, Viana JF, Sousa N, Maciel P, Pinto L, Oliveira JF*. (2021). IP 3 R2 null mice display a normal acquisition of somatic and neurological development milestones. Eur J Neurosci. Sep;54(5):5673-5686. doi: 10.1111/ejn.14724.
Sardinha VM, Guerra-Gomes S, Caetano I, Tavares G, Martins M, Reis JS, Correia JS, Teixeira-Castro A, Pinto L, Sousa N, Oliveira JF (2017) Astrocytic signaling supports hippocampal–prefrontal theta synchronization and cognitive function. Glia 65:1944–1960.



Contact us
Phone: +351 253 604 967
Fax: +351 253 604 809
Email: icvs.sec@med.uminho.pt
Address
Life and Health Sciences
Research Institute (ICVS)
School of Medicine,
University of Minho,
Campus de Gualtar
4710-057 Braga
Portugal

Copyright ©2022 ICVS. All Rights Reserved



Copyright ©2022 ICVS. All Rights Reserved
Address
Life and Health Sciences
Research Institute (ICVS)
School of Medicine,
University of Minho,
Campus de Gualtar
4710-057 Braga
Portugal



Copyright ©2022 ICVS. All Rights Reserved
Address
Life and Health Sciences
Research Institute (ICVS)
School of Medicine,
University of Minho,
Campus de Gualtar
4710-057 Braga
Portugal